Oisin Biotechnologies Produces Impressive Mouse Life Span Data from an Ongoing Study of Senescent Cell Clearance
Oisin Biotechnologies is the company working on what is, to my eyes, the best of the best when it comes to the current crop of senolytic technologies, approaches capable of selectively destroying senescent cells in old tissues. Adding senescent cells to young mice has been shown to produce pathologies of aging, and removal of senescent cells can reverse those pathologies, and also extend life span. It is a very robust and reliable approach, with these observations repeated by numerous different groups using numerous different methodologies of senescent cell destruction.
Most of the current senolytic development programs focus on small molecules, peptides, and the like. These are expensive to adjust, and will be tissue specific in ways that are probably challenging and expensive to alter, where such alteration is possible at all. In comparison, Oisin Biotechnologies builds their treatments atop a programmable suicide gene therapy; they can kill cells based on the presence of any arbitrary protein expressed within those cells. Right now the company is focused on p53 and p16, as these are noteworthy markers of cancerous and senescent cells. As further investigation of cellular senescence improves the understanding of senescent biochemistry, Oisin staff could quickly adapt their approach to target any other potential signal of senescence - or of any other type of cell that is best destroyed rather than left alone. Adaptability is a very valuable characteristic.
The Oisin Biotechnologies staff are currently more than six months in to a long-term mouse life span study, using cohorts in which the gene therapy is deployed against either p16, p53, or both p16 and p53, plus a control group injected with phosphate buffered saline (PBS). The study commenced more than six months ago with mice that were at the time two years (104 weeks) old. When running a life span study, there is a lot to be said for starting with mice that are already old; it saves a lot of time and effort. The mice were randomly put into one of the four treatment groups, and then dosed once a month. As it turns out, the mice in which both p16 and p53 expressing cells are destroyed are doing very well indeed so far, in comparison to their peers. This is quite impressive data, even given the fact that the trial is nowhere near done yet.
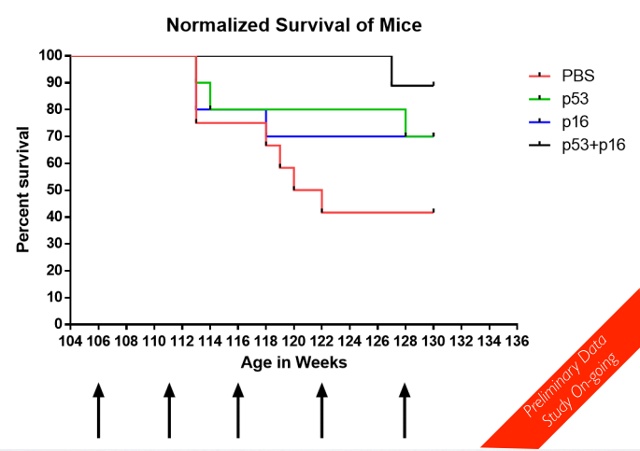
The image presented here is taken from the Oisin Biotechnologies PDF that accompanies a presentation given at the recent ICSA meeting in Montreal. We should certainly hope to see more of this sort of thing in the years ahead as senolytic technologies improve.
Whoa.
Am I reading this right? That this study is still ongoing? That 90% of the p53 and p16 mice are still alive at 130+ weeks?
This is the kind of data that Aubrey is talking about when he says that 'Soon everyone will know that this is possible'.
Raise a glass my friends, we have made a LOT of progress this year.
That is remarkable data
Data is starting to speak and it's telling Aubrey he was right on the money !
Kudos to the excellent team at Oisin and everyone who supported them, including you Reason and the Methuselah Foundation.
HOPE
Hello everyone. Does anybody knows what PBS on the graph stands for? Does it for placebo?
ph buffered saline solution, so yes a placebo
Are these mice genetically engineered to secrete something from p53/16 cells that therapy targets, or are they wild type?
What shall be really telling when the study is complete, is the effect on maximum lifespan.
@Mark: Standard issue C57BL/6 wild type lab mice.
The Methuselah Mouse record for now is about 260 weeks. By definition, we will need two and a half more years beat it using p53+p16.
What is remarkable that the control group (PBS) has flat mortality after week 122. In fact , no mortality at all. I guess the ones surviving 122 have the longevity genes. If we compare weeks 112 to 118 we have a precipitous drop in the survivability , about of 50% of all the living individuals at week 118!!!
Another interesting observation is that the drop of p53+p16 on week 127 is almost the same as the first drop of p16 on week 114. It is like p53 postponed something that p16 could not handle but it has came back 13 weeks after.
Probably the sample size is small and such artifacts could happen. And my observations could be akin the technical trading :)
One point to keep in mind - these are preliminary data. When the study ends my personal prediction is we may not see much max LS extension, but rather a fairly significant median LS improvement. That's intuition, not analysis.
Subsequent to this presentation, we have seen additional mortality (one p53-treated and one additional control mouse dead) but no further change in the p16 or combo cohorts. We are currently at 6 treatments and just over 135 weeks.
Hi Gary,
thanks for the update!
Can you guess the shape of the mortality curve for the controls from other studies?
> my personal prediction is we may not see much max LS extension
So would you expect a sharp decline at the end, which would lead to almost square shape of the chart ?
@Gary,
will you not expect at least the same maxLS extension as from calorie restriction ?
@cuberat: I'm assuming "squaring of the curve" similar to what the Mayo team saw in the 2016 Nature paper. I don't want to speculate on max LS for at least another 6-8 months.
Hi Gary,
how soon do you think human trials could start, assuming all goes well with the mice and other animal models?
From the report I read that "Forty aged mice were randomly put into these groups ". It would be interesting to know how many mouse originally were in each group? Did each group have the equal number of mouse, 10 mouse in each group or not?
cuberat: flat regions of mortality are not surprising. This is a survival graph, which means that each time a mouse dies, a line drops; if you count the number of drops in the control line, there's apparently ~5 mice left in that flat part. With n=10 in each arm, that's just not a lot of mice which *could* die and make it wiggly. Flatness is inherent to the sample size & method of visualization.
(Observing any difference in median survival will be difficult with small samples, and I'm not sure that this would be able to answer any more subtle questions like differing shapes of curves... Survival analysis requires a lot of samples, nonparametric approaches usually require more samples, and post hoc questions like 'does this part wiggle differently from that part in the control' require even more.)
I will be surprised to see this work out much differently in general shape than the recent dasatinib/quercetin study mouse mortality charts, in which the descending slope of survivor count for treated and untreated cohorts were parallel to one another all the way down:
https://doi.org/10.1038/s41591-018-0092-9
The experimental designs are similar. The question is how far right does the treated group move on the time axis before it starts the decline, which I'd expect to reflect the quality of the senolytic, given enough mice to dampen down the law of small numbers.
I wish they would have double blinded the study. Is it common to not double blind these kinds of studies? It wouldn't raise the cost of the study by much.
@Bruce: Death is a pretty binary readout, so we didn't need to blind the study. No subjectivity is required...
@Reason
>which I'd expect to reflect the quality of the senolytic,
It would also depend on how genetic or specific the exact senolitics are. Also there might be more pathways that prevent apoptosis. In this case the senolitics will remove point a fraction of the senecent cells. It wood be too simple and too easy if there were only p16 and p53.
Wouldn't initiating cell apoptosis based on an activated p53 pathway also reduce incidence of death from straight cancer in the mice, not just from senescence (yes, which does cause cancer along with a whole lot of other ailments of aging)?
YES!! Congratulations to Oisin's staff and all its supporters!
For Gwern, "This is a survival graph, which means that each time a mouse dies, a line drops; if you count the number of drops in the control line, there's apparently ~5 mice left in that flat part."
But the red line drop at week 113 is huge, I suggest this line drop represents 2 mouse deaths. So in my opinion, after 123 the red line points at 40 % in survival axis, which means 6 mouse died and only 4 left.(Each mice death is represented by 10% line drop). Alternatively I would suggest that the graph is very schematic and was drown not in scale.
Gary said he expects a squaring the curve effect, with little effect on mouse max lifespan. I agree this is likely as nothing is being done to replace those ablated cells using a telomerase/stem cell treatment. It would be nice to be surprised however.
I'm wondering if Oisin have in fact solved the long standing safe, efficient, and repeatable delivery problems of gene therapy?
A quick google search reveals Entos Pharma who seem to be licencing the technology for use in regular gene therapy.
From what I understand the fusogenix protein causes the lipposomes to fuse with all cells, is there a way to target specific cells types selectively?
Mark,
>Gary said he expects a squaring the curve effect, with little effect on mouse max lifespan. I
I would expect it to have similar effects as calorie restriction. Move have high Max LS plasticity. Humans, on the other hand don't.
However, the mediant health span gain might be quite huge.
I don't agree Cuberat. Calorie Restriction and Senescent cell removal are quite different beasts. The former will slow cellular senescence, the latter just removes them, without impacting their rate of accumulation (note - SASP induced senescence will be reduced, but more cells will need to divide to replace those ablated; it is an open question whether this will slow down or accelerate senescent cell creation).
@Jim: On cancer, yes, you'd expect the p53 targeting to reduce cancer rate significantly. That's a big deal in mouse life span studies because their cancer rate is high. You might recall the debate over whether rapamycin actually slows aging across the board versus only reducing cancer incidence. Determining the difference between those two things in mouse studies isn't straightforward.
Good article on Singularity Hub:
https://singularityhub.com/2018/07/17/this-drug-combo-extends-lifespan-and-healthspan-in-mice-by-killing-zombie-cells/
I'm wondering if CD38 protein levels could be used as a valuable biomarker of senescence in these mice studies, as it is known to increase in mammals during aging, representing a decline in NAD+ metabolic levels with increasing age.
If Gary is still around, can he comment on the health of the treated mice? Are they robust and vigorous? Are they doing better than the untreated ones?
I don't have quantification of robustness, but they seem healthy. By the way, in response to the question about cancer rate - the Nature 2016 paper reported a 50% reduction in cancer incidence in treated animals (p16, they didn't target p53). So far we have not seen cancer deaths in our treated animals, to my knowledge. Someone also asked about group size - 12 animals in PBS, 10 each in p16 and p53 and 9 in combo. Just the way the randomization came out.
I'll caution patience once again. The study will run until all animals die, and then we have to publish, so we still a long way from a peer-reviewed paper. Meanwhile, we'll be seeking collaborations for replications, moving into other mammalian model organisms, and refining the platform as we move to Phase 1 clinical trials for solid tumors in 2019.
For Gary and everybody please. How many mouse from the control PBS group is left in week 130? According the graph the red line points at 40 % on axis of survival by week 130. Every 10 % drop represents death of 1 mice. We can clearly see 5 red line drops by week 130. Originally the BPS group (the red line) has 10 mouse. Is this correct? Many thanks for clarification.
@Aleks: see my post above. We had 12 animals in PBS group; as of today (~136 weeks) 4 are surviving. The graph above reflects survival of 5 animals. Each death in PBS is a drop of 8.3% in PBS only, 10% in p16 or p53 and 11% in combo.
It would be very interesting to see this combined with other methods like partial reprogramming, rapamyacin, and stem cell replacement
@Chris,
This approach seems to work quite well alone, and since it uses a different target might have nice synergistic effect with some other approaches.
* Rapamycin/Sirolimus as mTOR inhibitor enhances the apoptosis. It remains to be seen if the combination of p16+p53 OISIN treatment will be compounding or slightly enhancing. I would rather expect both of them to be targeting similar pathways, so no much compounding here. But still could boost the senolitic action
* stem cell replacement. It might turn to be much harder, since most stem cell injected don't stay for a long time. There we are still scratching the surface. On another hand, it might be that even at 120 years there enough stem cells, as long as you get rid of the inflammation... So I would say stem cells depletion to be less immediate problem
* partial reprogramming - if you mean factors and and such, I would say that it could have an independent work. For generic reprogramming... well we are not there yet...
@ Gary. Many thanks for your explanation. Now the data on the graph makes to me a sense. I'll importunately wait the final result of the experiment. Please keep us update.
Very promising, thanks for sharing. Especially promising factoring in one additional control has died in the past five weeks since the presentation. I calculate 4 of 12 controls still living, 8 of 9 in the combination treatment group still living after 135 weeks. The mice in the combination treatment group will have to start dying really soon to make the argument maximum lifespan has just been squared off but not extended
@John D
The squaring of the curves means that maximum lifespan status is almost the same while the average and median can grow significantly. That's what we witness with people. The life expectancy grows yet the max LS stays more or less the same. However, mice have more elastic Max lifespan.
Statisticalky speaking , for adult humans the mortality doubles roughly every eight years(Gompertz law , simple doubling can lead to probability more than hundred % , but it can be replaced with the probability of survival in the form of p=~exp (2t)). So, at 100 years the probability of dying at that age is 1024 times more than at the age of 20 ( green doublings 2^10=1024,). This formula gives a nice sigmoid curve. However, with various lifestyle and medical improvements we can reduce the chance of dying at the age 50-80, even 90. However, after that the survival rates are plummeting, which leads to curves that have more square shape. Which means that all these medical improvments don't make us any younger younger nor slow the aging, they merely let us linger longer to get even older and have a higher chance of dying. (And need even more and a pricier medical attention)
Here is an example of squaring curves:
https://ourworldindata.org/wp-content/uploads/2017/08/Survival-Curves-UK.png
https://ourworldindata.org/life-expectancy
So in theory of there is a treatment that can increase the survival across all ages and shift the whole survivability curve right we can speak of de-facto statistical rejuvenation or delay /slowdown of aging.
But all this fancy curves aside , even if you increase only the health span , it is already huge.
@Cyberat I know the broad stokes as to what squaring the curve means. No doubt there are details such as extrapolating data from small sample sizes I don't know, or really need to know if the data is robust enough.
My point was, given the current mortality rate of the controls, all the controls will likely be dead by week 155 or 160. And if there are still 5+ mice from the combo treatment group alive by say week 165, I think one will be able to say with a high degree of confidence the combo treatment extended maximum lifespan (for mice). Without having to wait an unknown number of weeks for all the mice to die and all the numbers to be crunched.
We always say that human maximum lifespan is not elastic, unlike mice, but I wonder if this is really true.
As Cuberat points out, medical technology has already 'squared the curve' for humans - so this, I think, is probably why calorie restriction and other metabolic interventions seem to only improve healthspan and not lifespan: we already have banked those years (in various states of ill health) due to healthcare, which mice do not have access to.
At Zoo near where I live they have a penguin that's 40 years old and still fertile. She has Vets on call and is given anti inflammatories daily. Bet she wouldn't live much longer with CR either.
John D, Mark,
I have found a survival curve from a calorie restriction study. There the time is given in months, though.
http://www.lifeintherightdirection.com/wp-content/uploads/2016/12/mice-calorie-restriction.gif
The control group dues out song 36 months. Let's say 3 years. 3x52=156 weeks. Or about 20 more weeks for the Oisin study. But even with the current chart in the article the results are astonishing. The results so far are as good as the extreme CR. We can do some extrapolations. CR and p15/53 have different mechanisms , so now I will do some guesstimates. CR had broader metabolic effect. It promotes apoptosis and reduces the damage buildup, and can reduce the inflammation even if it is not caused by senescent cells. P1653 is very targeted. It doesn't reduce the blood sugar on its own, but it's more effective in removing the senecent cells. I would say that at early to mid life CR might be more effective (just got feel), while senecent cells ablation will be easy more effective at later stages when the cleanup systems are compromised. Therefore, I would expect similar results with CR till wrek 180-200 and better survival after
@Reason:
How do you think all these treatments will be commercialized? For example by selling patents? Opening clinics all over the world? By franchises? Another? I mean, if we want to get involved as a seller, how do you think it will be possible?
Thanks!
@JohnD, @Cuberat:
What Reason and others say is not that human lifespan is not so elastic as mice lifespan, but that it's not so elastic as mice lifespan for the treatments usually used to extend lifespan in mice, that is, calorie restriction, exercise mimetics, rapamycin and the other "messing with metabolism" approaches. Those approaches work worse for long lifespan species because all of them are more or less evolved adaptations to survive famines, and thus provide at most a couple of years of life extension, independently of the species, because longer famines are just to rare to develop adaptations to them.
OTOH, damage repair approaches don't rely on evolved, natural life extension mechanisms that the subjects are born with. Thus, the amount of the extension is not restricted by that couple of years.
@Josep: The usual path of selling the developing company to Big Pharma, that then finalizes the clinical trials and markets the therapy to physicians for specific diseases of aging.
The question is the degree to which that usual path will be augmented, as happened for stem cells, by clinics outside the primary regulatory environments. I think it will be much more prevalent for senolytics, given how cheap the initial candidate drugs happen to be.
How to get involved as a seller, see the ideas in:
https://www.fightaging.org/archives/2018/07/thoughts-on-the-ending-age-related-diseases-conference/
If it seems too good to be true, it probably is.
Gary, can you update us on the surviving population? I think you were at 4/6/7/8 for PBS/p53/p16/combo in mid-July? Thanks!
I got an update on the surviving mouse population at week 140 in an interview with Stephen Hilbert (Director of Head of Corporate Strategy/Development Pre-Clinical Oisin Biotechnologies and OncoSenX): http://www.scifuture.org/surviving-the-zombie-cell-apocalypse-oisin-biotechs-stephen-hilbert/
Wow, what's the problem with p53?
Thanks , Adam
Alas the sample size is too small to make any definite conclusions.
If the p53 drop turns out to be real, the explanations cold be numerous... Like being to toxic late at life and need trying the protocol.
But yeah. P53 target alone send to square the life expectancy curve. And the combination finally postponed the mortality by at least 30 weeks.
So this detoxification protocol while impressive is not our miracle. But if it translates well in people, can buy you 10-30 more years of healthpsan. ... Count me in !
Hello, using about 10 mice per group is VERY LITTLE. Mouse lifespan tests typically use n=25 per group at least to statistically demonstrate good-but-non-giant-effects (http://agevivo.com/stats/LOLES.html).
At this stage (I checked and you can reproduce it just here below), there is NO statistical significance it could quite be random luck for now. Unless the treated mice show a nice maximum life extension, the results may barely be of statistical significance ==> @Oisin team, suggestion that you start at least another round of mice in parallel, without waiting more!!!
Explanation:
- looking at the curve there are 12 control mice, 10 p53 and 10 p16 mice and a priori 9 p53+p16 mice.
- it is statistically not unlikely that the treatments have no effect (probability 23.4%):
noting 1 the control, 2 the p53, 3 the p16 and 4 the combination group, this is the code in R for a logrank test:
library(survival)
survdiff(Surv(c(
113,113,113,118,119,120,122,130,130,130,130,130, #tPBS
113,114,128,130,130,130,130,130,130,130, #tp53
113,113,118,130,130,130,130,130,130,130, #tp16
127,130,130,130,130,130,130,130,130 #combination
)-104, c(
1,1,1,1,1,1,1,0,0,0,0,0, #PBS status
1,1,1,0,0,0,0,0,0,0, #p53 status
1,1,1,0,0,0,0,0,0,0, #p16 status
1,1,0,0,0,0,0,0,0 #combination
)) ~ c((1:12)*0+1,(1:10)*0+2,(1:10)*0+3,(1:9)*0+4), rho=0) #we get p=0.234
Comparing just PBS and the combination leads to p=6%. Doing many comparisons like this would require a low p (5%/n tests) to be convincing.
survdiff(Surv(c(
113,113,113,118,119,120,122,130,130,130,130,130, #tPBS
127,130,130,130,130,130,130,130,130 #combination
)-104, c(
1,1,1,1,1,1,1,0,0,0,0,0, #PBS status
1,1,0,0,0,0,0,0,0 #combination
)) ~ c((1:12)*0+1,(1:9)*0+4), rho=0) #we get p=0.0598
All the best for so interesting experiments.
Regarding that human lifespan is not so elastic as mice lifespan... perhaps, perhaps not.
A lot of this notion comes from the fact the c elegans lifespan has been increased 10 fold and that two well known primate CR studies yielded poor life extension message. However:
-- c elegans is quite special for life extension (with its dauer phenotype) and has had so many more tests than mice that it is difficult to partially conclude at all
-- the US primate CR experiment were quite questionable as the species used tend to mutilate themselves and as it has been seen in various strains of mice, CR does not always extend lifespan in mice, it seems very much linked with how well mice tolerate CR (notably how fast CR is turned on, at what age, etc), and the US experiments suggested good health results for animals that didn't mutilate themselves
-- a 2018 paper about CR in Lemurs shows a 50% life extension (from 6 to 10 years) while reducing cancer rates by 5 fold: (Pifferi et al. 2018). It seems to me that this study is being ignored due to a lack of advertising compared to the US studies, but it deserves probably the same advertising.
Regarding humans, some people argue that there is no superlongevity found in humans, perhaps this is true, but without looking far there is an interesting male-female difference, and -something I never disclosed but I do it here- there is a **strong neurodegenerative aging rate** in humans (that starts very low at young ages) so as soon as we have strong therapies against Alzheimer's and Parkinson's we can unleash other improvements on general aging.
Are the mice still alive?
I have heard the control group is already dead but a few of the treated mice are still alive and well. No details and no update on the survivability graph, though. I was expecting OISIN to publish an update somewhere in January. There might be some not- disclosure agreement that doesn't allow them to publish it before the study is finished, or something...
But due to the small group size the results could be questioned if the effects is small..